MRI Physics: Cardiovascular Pulse Sequences
This page discusses cardiovascular MRI pulse sequences. You should first review standard MRI pulse sequences as well as flow-related phenomena in MRI.
Black Blood
Black blood, also known as dark blood, sequences are designed to null signal from flowing blood and highlight static anatomy. They may also be used, in combination with an inversion pulse, to look for myocardial edema.
As the name implies, in black blood sequences we want flowing blood in vessels or the heart to be black (nulled). As you may recall, in a standard spin echo sequence, flowing blood tends to lose signal - producing a flow void. This is because blood flowing into the plane is not present for both the 90- and 180-degree pulses, therefore its signal will not recover for the spin echo like static tissue (which does experience both pulses). The degree of signal loss is directly related to the speed of blood flow. For cardiovascular purposes, we often want to produce truly black blood, even with slower flow.
In order to do this, we will employ a double inversion recovery sequence. You may want to review the principles of inversion recovery sequences before continuing. For double IR, we first invert protons from the entire volume of tissue (i.e. entire chest). Immediately afterwards, we apply a second inversion pulse that is slice-selective - this returns protons from our desired slice back to normal. We then wait for an inversion time TI until blood in the volume reaches zero magnetization. At that point, any blood flowing into the slice will have black signal. We then start our acqusition, which is typically a fast spin echo (usually half-Fourier sequence, also known as HASTE or RARE).
Double inversion recovery has an advantage over a simple inversion recovery sequence for nulling blood because the T1 of blood is very similar to myocardium. So if we used a simple IR sequence, much of the anatomy we would like to see would be black! By appling whole-volume followed by slice-selective inversion pulses, we can restrict the inversion recovery nulling only to blood flowing into the slice and therefore avoid nulling myocardium within the slice.
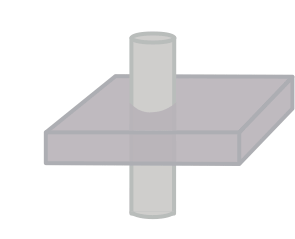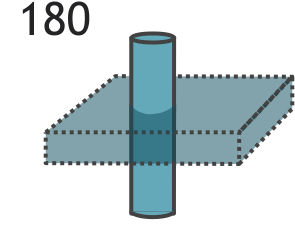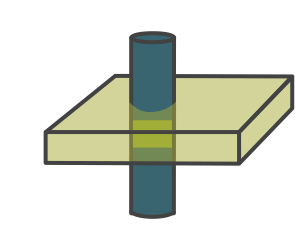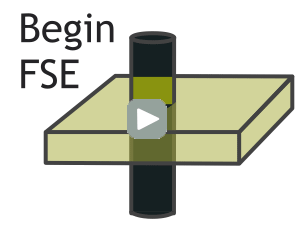
Illustration of the double IR black blood sequence. The initial 180-degree inversion pulse inverts the entire volume of tissue, and this is followed by a slice-selective inversion pulse. That pulse restores normal signal to the slice we are imaging. The standard spin echo sequence begins after blood in the volume has reached its null point.
The inversion time TI is set to null any blood that may flow into the slice and so depends on the T1 of blood. As discussed in the article on inversion recovery, the inversion time TI also depends on the TR of the sequence. The TR is set by the patient's heart rate, and so TI does depend a bit on the heart rate. Any blood that remains within the slice, of course, does not get nulled. This is a problem with slow-flowing blood (e.g. in a left ventricular aneurysm) as well as any blood that flows within the plane (e.g. sagittal image of the aorta).
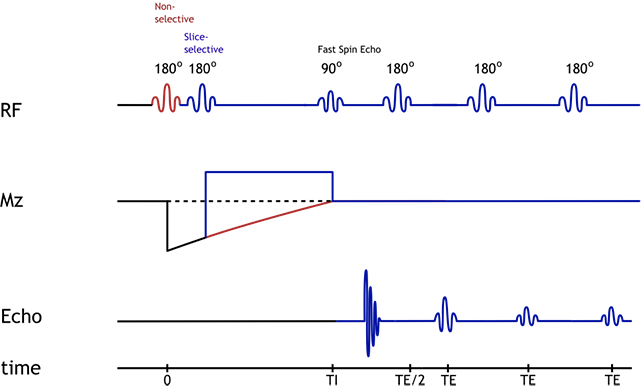
Double IR Black Blood pulse sequence diagram. Blue indicates signal/protons within the slice, while red indicates protons within the remainder of the volume.
Triple Inversion Recovery. Double IR sequences are generally T2-weighted (if they are acquired with half-Fourier FSE techniques such as HASTE). Therefore, they are somewhat sensitive to myocardial edema. However, fat remains bright, and myocardium tends to have intermediate signal. In order to accentuate signal from myocardial edema, an extra inversion recovery pulse may be added to null fat, thus obtaining a STIR (short tau inversion recovery) image with black blood. This third inversion pulse is slice selective and is added just before the 90-degree pulse in the FSE part of the black blood sequence.
Bright Blood
Bright blood sequences, in which flowing blood retains signal, are used to evaluate vascular flow and cardiac function. These may be used as part of a non-contrast MR angiography protocol as well.
Blood is typically bright on T1 and T2 weighted sequences. However, flow and turbulence reduce the signal from intravascular blood. Therefore, for cardiovascular applications, we need to employ flow compensation gradients.
In the past, regular gradient echo sequences (e.g. FLASH or TurboFLASH for Siemens) were used to produce bright blood images. By adding flow compensation gradients to the GRE, blood would remain bright. The advantage of using GRE sequences is that they are fast - fast enough to obtain cine (movie) images of cardiac motion. These GRE sequences are 'spoiled', meaning that residual magnetization is destroyed at the end of each pulse cycle. The disadvantages of spoiled GRE sequences are: (1) relatively lower signal than SSFP (see below) and (2) accelerating and turbulent blood lose signal. The latter effect is caused by the fact that flow compensation gradients cannot fully compensate in those situations.
Currently, most cine bright blood imaging is produced using balanced steady-state free precession (SSFP) sequences, e.g. TruFSIP for Siemens or FIESTA for GE. In SSFP, instead of spoiling or destroying longitudinal magnetization at the end of each cycle, it is refocused so that it contributes to the signal in the next cycle.
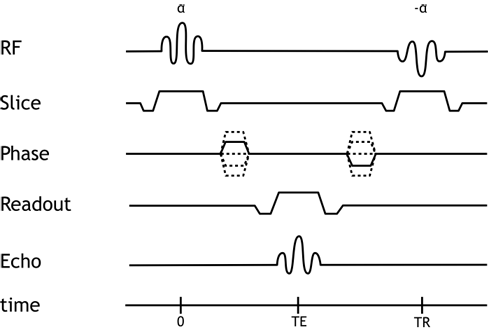
Pulse Sequence Diagram for the balanced SSFP sequence.
This refocusing provides the advantage of much better signal in the images. Additionally, the refocusing or balancing gradients that are used in SSFP have the added benefit of providing flow compensation that is better than that in spoiled GRE sequences. Tissue contrast in balanced SSFP sequences depends on both T1 and T2*, with fat and blood being relatively bright. SSFP sequences are extremely fast, making them ideal for generating high temporal resolution images of the moving heart. The disadvantages of SSFP sequences include: (1) relatively less sensitivity for turbulence, which can mask subtle jets from regurgitation, leaks, or septal defects; and (2) greater sensitivity to magnetic field inhomogeneity. Additionally, SSFP sequences may suffer from a variant of the vascular ghost artifact. In large, high-velocity blood vessels (particularly the aorta), one may see artifacts propagating along the phase-encoding direction in systole. This is because the flow compensation works so well in SSFP sequences that even protons that have passed out of plane may contribute signal to the image (but be misregistered). If the artifact is particularly troublesome, one may wish to switch to a spoiled gradient echo cine sequence, which does not suffer from this phenomenon.
ECG Triggering and K-Space Segmentation
One item we have neglected is the actual mechanics of acquiring these sequences in sync with the cardiac cycle. Even with relatively fast pulse sequences, generally we cannot acquire the entire image in one diastole. Thus, similar to cardiac CT, we need to employ ECG gating to identify the phase of the cardiac cycle and acquire data in the relevant portions.
Black Blood. In black blood sequences, typically the entire slice can be acquired in a single diastole (using half-Fourier FSE techniques). Generally we do want to obtain this in diastole to minimize cardiac motion within the slice, however. Also, if we trigger the first inversion pulse around systole, we can image at the point of peak blood velocity, which improves our ability to null the signal from blood (since it's moving faster through the slice).
Bright Blood. In bright blood sequences, we generally wish to obtain moving pictures (cine images) of the heart. In order to do this, we must acquire data throughout the cardiac cycle. The MR scanner will use the ECG signal to divide the cardiac cycle into blocks, e.g. 8 per cycle. The scanner can then calculate how much time it has within each block and therefore how many lines of k-space it can acquire per block per cycle. Imaging is then done over several heartbeats (often 5-8 beats), segmenting k-space into groups of lines that are acquired in each heartbeat. Lines of k-space are then assembled for each part of the cardiac cycle, and the Fourier transform is applied to recover the image from k-space.
Alternatively, we can attempt to acquire bright blood images in real time. This requires decreasing the spatial resolution (number of lines of k-space) so that we can acquire the entire image within each block of the cardiac cycle. Real-time imaging may be used in the 'sniff' maneuver to look for ventricular interdependence in constrictive pericarditis, for example. It may also be helpful in patients who have difficulty in breath-holding.
Delayed Enhancement
In order to image myocardial inflammation or fibrosis, we image the heart approximately 10 minutes after administration of a gadolinium contrast agent. Normal myocardium enhances, but abnormal myocardium retains contrast even more. To improve the contrast between these two degrees of enhancement, we can employ an inversion recovery sequence to null signal from normal myocardium.
While the inversion time (TI) for normal myocardium is generally around 300 ms, it is necessary to determine the optimal TI empirically for each patient, since this will vary a bit from person to person and over time as gadolinium washes out. A "TI Scout" sequence is performed just prior to doing the IR imaging, looking at a single slice of the heart. This sequence generates a set of images at varying TI times. By looking at the image, the radiologist or technologist can choose the optimal TI time that nulls normal myocardium (makes it totally black). This will give the optimal contrast between normal myocardium and bright (enhancing) abnormal myocardium. The pulse sequence used to generate the TI scout is rather complicated and beyond our current discussion; it is typically a Look-Locker sequence.
Sometimes it can be challenging to select a TI time if the myocardium is diffusely abnormal, such as in cases of amyloidosis. Additionally, the optimal TI time varies over time as contrast slowly washes out of the myocardium. This can yield confusing, washed-out, or even frankly non-diagnostic delayed enhancement IR images. This problem led to the development of the phase-sensitive inversion recovery (PSIR) sequence, which avoids the need to choose TI so accurately.
Phase-Sensitive Inversion Recovery. As described in detail in the article on inversion recovery sequences, the 180-degree pulse makes the net magnetization initially negative. The net magnetization recovers in the vertical axis to eventually point towards B0, the process of T1 relaxation. In IR sequences, the 90-degree imaging pulse is chosen to coincide with when the desired tissue is nulled, or passes through zero net magnetization; this is TI. If TI is chosen too early or late, net magnetization will be non-zero (either negative or positive, respectively). When this magnetization is rotated into the transverse plane, the scanner has no way of knowing whether it was initially negative or positive. In fact, this information can be inferred by the phase of the rotation in the transverse plane; however, as discussed in spatial localization, we cannot directly measure the phase of a signal.
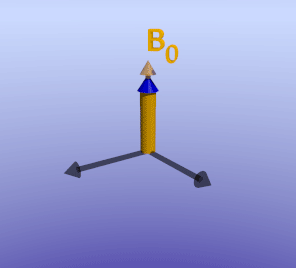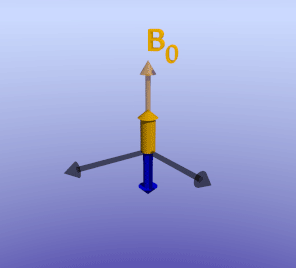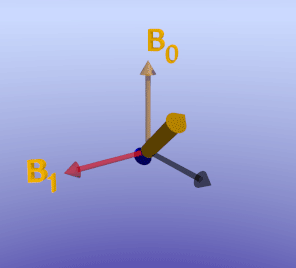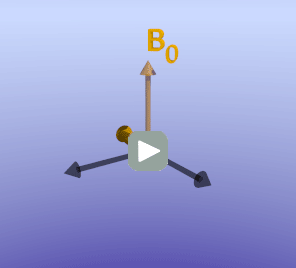
Illustration of an inversion-recovery sequence with sub-optimal TI time. The large arrows denote net magnetization of two separate voxels (or tissues). The orange arrow represents an area of delayed enhancement with more retained gadolinium and therefore faster T1 recovery, while the blue arrow represents normal myocardium with slower T1 recovery. Note that since the TI is not optimal, there is residual longitudinal magnetization in the blue tissue, which is flipped into the transverse plane by the 90-degree excitation pulse. The phase (direction) of the orange and blue arrows differs in the transverse plane.
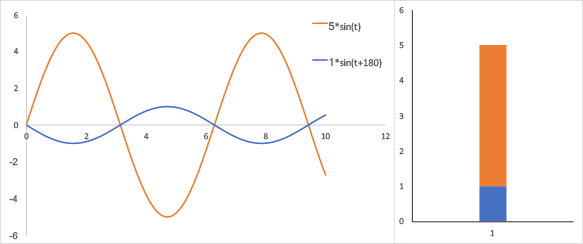
Signal in the tranverse plane from the illustration above. Left, oscillation of the net magnetization in the transverse plane; the signals are out of phase by 180 degrees; Right, Fourier transform of this system shows that the two signals overlap in the frequency domain. While the blue and orange signals are 180-degrees out of phase, the Fourier transform cannot distinguish that. The difference in amplitudes (signal-to-background) is 5 vs 1.
Phase-sensitive inversion recovery (PSIR) sequences take into account the phase - thus, whether the magnetization vector was positive or negative. They do this by subtracting the phase at the TI time from a reference timepoint. In many ways, this is similar to how phase contrast MR angiography works. More specifically, the PSIR sequence samples signal at 2 timepoints: one, at time TI; and the second at a much later time when magnetization has nearly completely reocvered to baseline. This is typically done over 2 successive heartbeats (per segment). The image data from the first timepoint can be used to generate a typical IR image (so-called "magnitude" image). Using the signal data in k-space from both timepoints (basically subtracting one from the other), one can keep track of the phase; the subtracted data is passed through the usual Fourier transform to generate the PSIR image.
| Magnetization Recovery | Magnitude of Magnetization Recovery | Magnitude PSIR |
| TI: 200 400 ms |
Simulation of magnitude and phase-sensitive inversion recovery sequences. Left, the longitudinal magnetization of the normal myocardium (blue) and infarct with delayed enhancement (orange) over time after an inversion pulse. Center, the magnitude (absolute value) of the longitudinal magnetization, which is what is measured in a traditional IR sequence. Note that there is a range of TI times where the difference between the orange and blue lines is quite small (approximately 217 ms - 280 ms). Right, simulated delayed contrast enhancement image using either Magnitude or Phase-Sensitive reconstruction sequences; the area of delayed enhancement (intended to represent a transmural infarct) lies in the inferior wall.
As illustrated above, the contrast on PSIR images is relatively insensitive to a mistake in TI. Traditional (magnitude) IR images are quite sensitive to TI, particularly if TI is too short. Remember that the correct TI varies over time, so if you are using magnitude IR reconstruction, you will have to adjust TI; this is not the case for PSIR.
A few minor points: (1) The subtraction process we referred to is a bit more complicated since it has to take into account phase effects from each individual coil. (2) The excitation pulses used in these sequences are typically much less than 90 degrees (these are, after all, spoiled gradient echo sequences).
References
- Ginat DT, et al. "Cardiac Imaging: Part 1, MR Pulse Sequences, Imaging Planes, and Basic Anatomy." Am J Roentgenol 2011.
- Ridgway JP. "Cardiovascular magnetic resonance physics for clinicians: Part I." J Cardiovasc Mag Reson 2010.
- Questions and Answers in MRI, "MRA Methods". MRIquestions.com
- Stemerman DH, et al. "Thoracic Aorta: Rapid Black-Blood MR Imaging with Half-Fourier Rapid Acquisition with Relaxation Enhancement with or without Electrocardiographic Triggering." Radiology 1999.
- Kellman P, et al. "Phase-Sensitive Inversion Recovery for Detecting Myocardial Infarction Using Gadolinium-Delayed Hyperenhancement." Magn Reson Med 2002.
Images and Content Copyright 2014 Mark Hammer. All rights reserved.